The CPAP solution that
We know what it’s like to live with sleep apnea. We also know how uncomfortable most CPAP masks can be. That’s why we developed a sleep solution that is comfortable, convenient and easy to use.
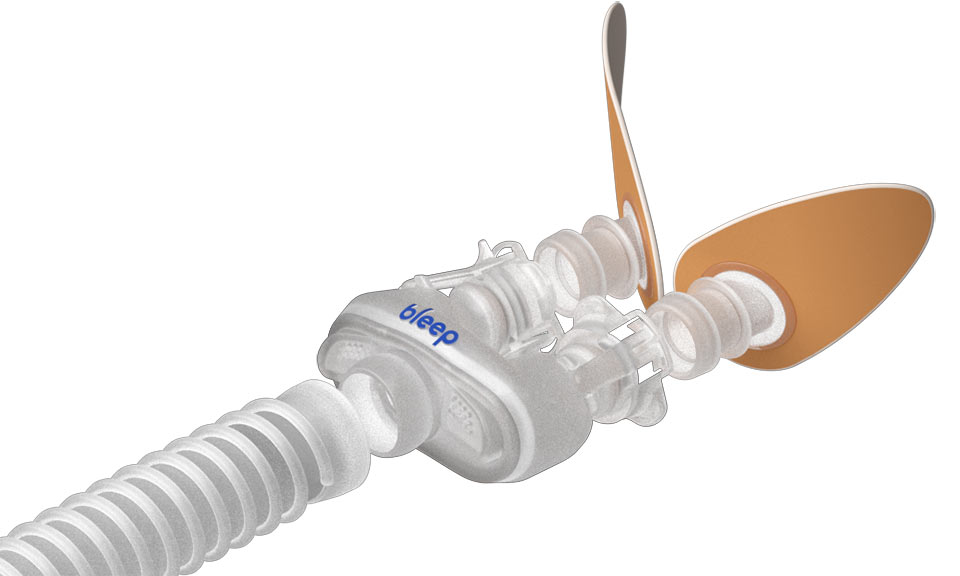
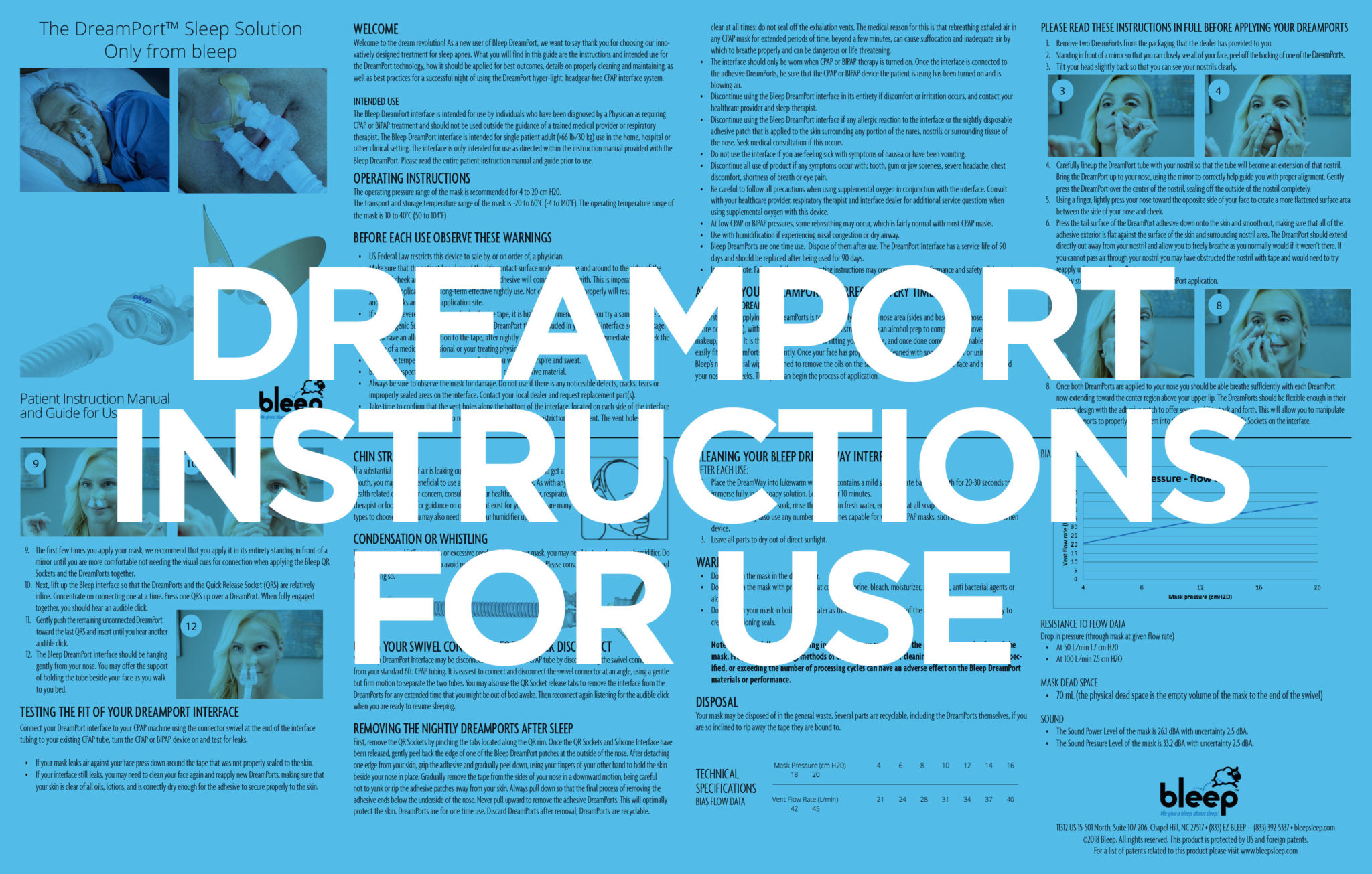
Our patented solution uses medical grade adhesive strips to hold the DreamPorts® in place under your nose for optimized comfort. Say goodbye to bulky headgear and uncomfortable straps – the DreamPort® Sleep Solution fits the exact shape of your nose to ensure zero leaks…just a great night’s sleep!
The DreamPorts® are easy to apply and won’t leave marks on your face at the end of the night. You can sleep on your back, side or stomach – the DreamPort® Sleep Solution won’t constrict your movement or get in the way. Get ready for quality sleep that will energize your day!
Key Features

Can be used before and during sleep
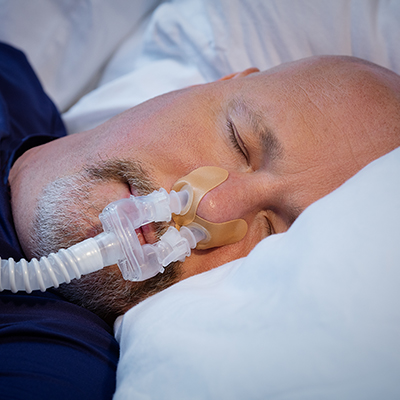
Can be worn comfortably with facial hair
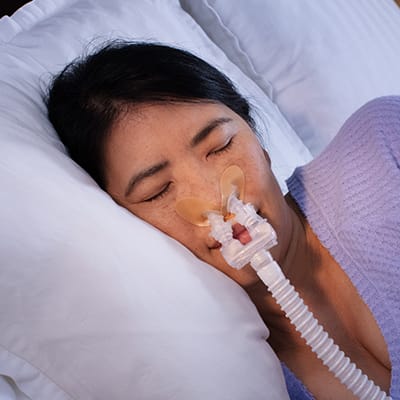
Lets you sleep on your back or your side

Lets everyone sleep soundly
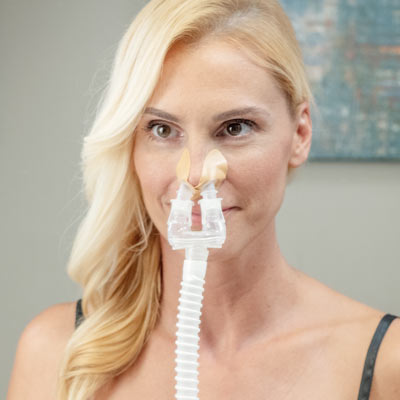
Application is quick and simple

Fits every nose shape perfectly
Instructions
CPAP tube management for sleep
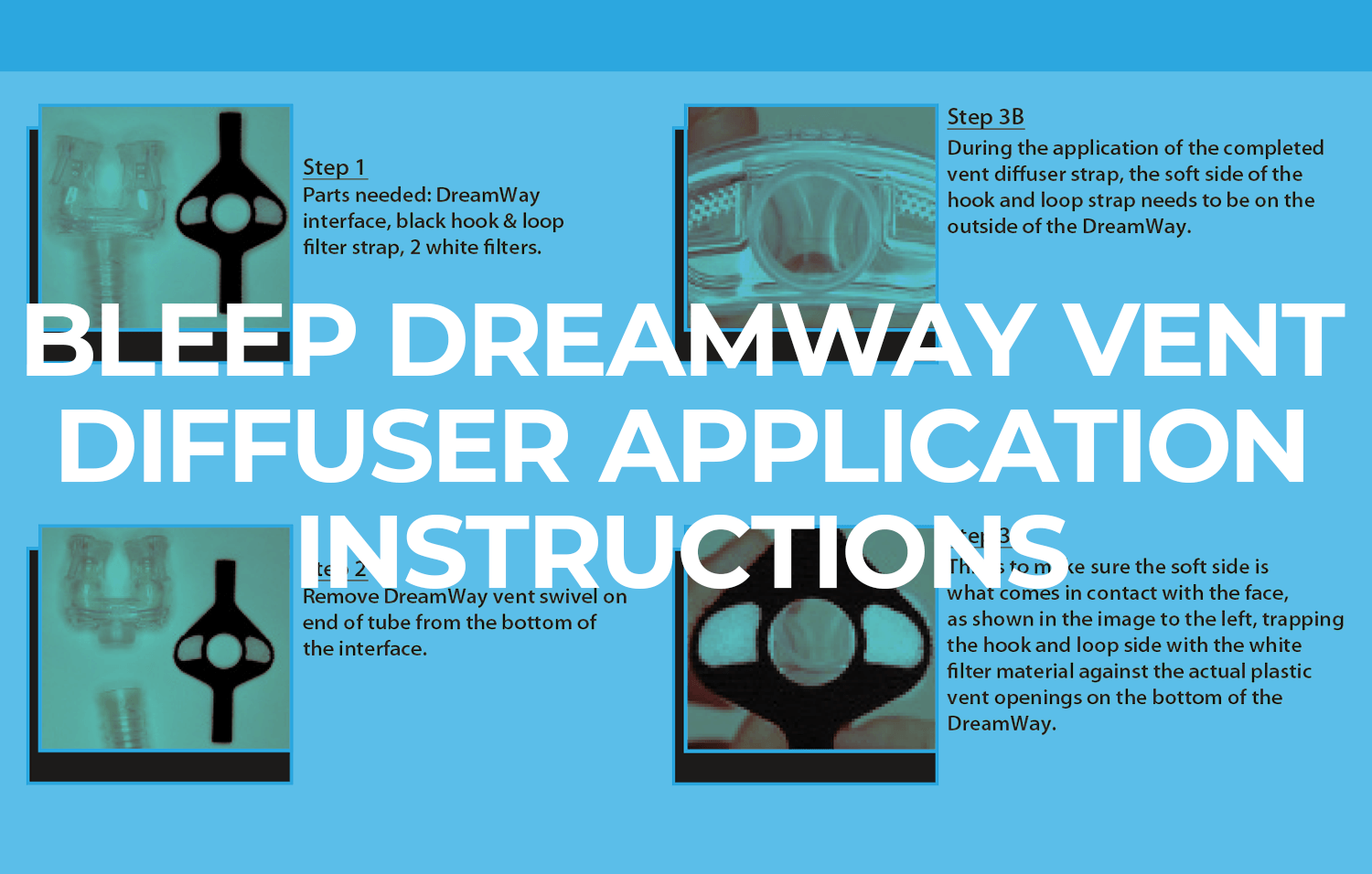
Customization of Your DreamPorts® for You
Make the mask a thing of the Past!
Is your CPAP mask waking you up at night?
Frequently Asked Questions
You’ve got questions, we’ve got answers.
What is the difference between DreamPort and Eclipse?
DreamPort® has a slightly lighter feel and requires more dexterity and good spatial orientation for application. It is also more flexible for upward-flaring nostrils that are positioned higher than the septum.
Eclipse™, on the other hand, is easier to apply and feels slightly heavier than DreamPort, though it is only 0.1 ounces more in weight. Eclipse is also slightly smaller and more compact under the nose, offering a more discreet fit.
Can I get a DreamPort® Sleep Solution today?
The Bleep DreamPort® Sleep Solution is available as of the week of March 4th, 2019! Please click on; WHERE TO BUY on the main page of our website.
Will the DreamPort® Sleep Solution work with my existing CPAP machine?
Yes. The DreamPort® includes a 10-inch connecting tube that connects to your current CPAP machine tubing and all CPAP machines. See our instructional video for example.
How long do the components last? (the DreamWay interface (mask) and the adhesive DreamPorts®)
The DreamWay user interface should be cleaned and dried after every use to prevent the risk of nosocomial infection (see cleaning video for detailed instructions). The DreamWay user interface is typically replaced every 90 days. The DreamPort® adhesives with connectors are a nightly disposable and 1 box of 32 will last for ½ of a month (1 set per day).
Will adhesives really stay on and do they hurt to remove?
The adhesives do work very well and are the only clinically proven solution not to leak. They are made of are made of gentle, surgical grade, PBA, Corn and Latex free soft tape – the same kind used on many patient applications in hospitals and homes. And no, it doesn’t hurt to remove the adhesives after use. (See instructional video for more information)
Are the DreamPorts® invasive? Do they go in your nose like nasal pillows?
No, the DreamPorts® are designed to meet at your nostril opening, but do not enter your nostrils like other nasal pillows.
How much will the DreamPort® cost?
The cost will be on par with your existing CPAP solution – following Medicare’s replacement schedule.
Is the DreamPort® Sleep Solution FDA cleared and do you have HCPCS coding from CMS?
Yes, we received FDA approval #K172335. We also have HCPCS coding; A7034 for Nasal Mask and A7033 for Nasal Pillow Cushions.
Is the DreamPort® available Internationally?
Currently, the DreamPort® Sleep Solution will only be available in the United States, but we will be considering International sales in the near future.
Financial Conflict of Interest (FCOI) Policy
Policy Statement
Bleep, LLC, is committed to integrity and objectivity in its research activities by defining and addressing potential and real matters of personal financial conflict of interest that may create or appear to create bias in the design, conduct, and reporting of research. Bleep, LLC, has implemented the following Financial Conflict of Interest (FCOI) Policy to address and monitor these concerns.
Reason for Policy
This policy and related procedures have been developed to identify, manage, mitigate, neutralize, or eliminate actual, apparent, and potential financial conflicts of interest. The policy was written to be in conformance with the Code of Federal Regulations (CFR) 42, Part 50, Subpart F, Responsibility of Applicants for Promoting Objectivity in Research for Which Public Health Service (PHS) Funding Is Sought[1] and 45 CFR Part 94, Responsible Prospective Contractors.
[1]These regulations do not cover Small Business Innovation Research (SBIR)/Small Business Technology Transfer (STTR) Program Phase 1 applications or awards but do apply to applicants and recipients under the SBIR/STTR Program Phase II. SBIR is the extramural research program for small business that was established by the Awarding Components of PHS and certain other Federal agencies under Pub. L. 97-219, the Small Business Innovation Development Act, as amended. The term SBIR Program includes the STTR Program, which was established by Pub. L. 102-564.
Applicability
All programs that apply for, or that receive, DHHS PHS funding through a grant or cooperative agreement for the purposes of research, and to each investigator (including but not limited to principal investigators, co-investigators, key personnel, consultants, other research staff, etc.) who participates, or are participating, in such research, including (including but not limited to principal investigators, co-investigators, key personnel, consultants, other research staff, etc.) participating through subaward agreements. For purposes of financial disclosure only, this regulation also covers the spouse and dependent children of the investigator.
Failure to Comply:
Failure to comply with this policy may lead to disciplinary or corrective action including but not limited to, removal from research project, suspending or terminating project, imposition of award conditions such as denying use of funds for parts of or all activities, suspension of funding, withholding further awards from project or program, and any other remedies that may be legally available.
Policy:
Bleep, LLC, has assigned a Designated Official to be responsible for the following:
- Informing Bleep, LLC investigators of their obligations under this policy and any related regulations;
- Reviewing disclosures of significant financial interest with Bleep’s Administrator to determine whether they are related to the subject research and, if so, whether they constitute financial conflicts of interest;
- Screening and managing potential financial conflicts of interest;
- Maintaining all records relating to disclosures of financial interests, Bleep’s review of and response to such disclosures, and any related actions under this policy;
- Ensuring inclusion of any required certifications in applications for funding or contract proposals; and
- Reporting and disclosure as required under this policy and applicable regulations.
For PHS-funded research, the Designated Official shall also have the following responsibility:
- Taking reasonable steps to ensure that Investigators for subrecipients (e.g., subgrantees, subcontractors, or collaborators) fully comply with this policy or provide Bleep with sufficient assurances to enable Bleep’s compliance with all applicable laws or regulations. To this end, the written agreement between Bleep and the subrecipient will specify whether Bleep’s or the subrecipient’s financial conflicts of interest policy will apply to the subrecipient’s Investigators and, if the subrecipient’s policy will apply, the Designated Official will:
- Obtain certification from the subrecipient that its policy complies with Bleep’s policy and the applicable regulations (absent such certification, Bleep’s policy will apply to the subrecipient’s Investigators, and
- Establish time periods for subrecipient reporting of financial conflicts of interest to Bleep that enable Bleep to report such conflicts in a timely manner, as required under its policy and the applicable regulations.
If Bleep’s policy will apply to the subrecipient Investigators, Bleep will be responsible for meeting the requirements of this policy and the reporting obligations reflected in the applicable regulations.
Internal Reporting Requirements
For PHS-funded research, as part of the funding application or proposal and prior to performing any work on the research, each Investigator who is planning to participate in the research is required by regulation to complete a Significant Financial Interest Disclosure (SFID) Form and submit the SFID Form to Bleep’s Administrator. This requirement also applies to Investigators who are or who work for subgrantees, subcontractors, or collaborators on PHS-funded research. SFID Forms will be provided to Investigators in conjunction with the annual training and will be otherwise made available. Bleep’s Administrator will review SFID submissions with the Designated Official. The information reported on the SFID Form includes a listing of the Investigator’s known significant financial interests and those of his/her immediate family that reasonably appear to be related to the research or that are in entities whose financial interests could be affected by the research.
Bleep Investigators in non-PHS-funded research who have any significant financial interest that may reasonably appear to be affected by the research are also expected to complete the SFID Form and submit it to Bleep Administrator.
Investigators are expected to submit an updated SFID Form during the period of the award as necessary (at least annually for PHS-funded research). The annual update will typically be done in conjunction with completion of the annual training. Such disclosures shall include any information that was not previously disclosed; any change in information regarding any previously disclosed significant financial interest; or, within 30 days of discovery or acquisition, any new significant financial interest (e.g., an interest acquired through purchase, marriage, or inheritance).
Determination and Management of Financial Conflicts of Interest
Upon receipt of a completed SFID Form, the Designated Official shall determine whether an Investigator’s significant financial interest is related to the subject research and, if so, whether the interest constitutes a financial conflict of interest under this policy and any applicable regulations. The Investigator may be required to submit additional information as part of the process. A disclosed interest may be related to the subject research either because the interest could be affected by the research or because it is in an entity whose financial interest could be affected by the research. A financial conflict of interest exists if the significant financial interest could directly and significantly affect the design, conduct, or reporting of the research.
If Bleep determines that a financial conflict of interest exists, a financial conflicts of interest management plan will be implemented and monitored on an ongoing basis. The management plan will include appropriate steps to manage, reduce, or eliminate the conflict. The following are examples of conditions or restrictions that might be imposed:
- Disclosure to research participants or the public of significant financial interests (e.g., when presenting or publishing the research);
- Monitoring of research by independent reviewers;
- Modification of the research plan;
- Disqualification of staff from participation in all or a portion of the research;
- Reduction or divestiture of a financial interest; or
- Severance of relationships that create actual or potential conflicts.
In addition to the conditions or restrictions described above, Bleep may require the management of conflicting financial interests in other ways as it deems appropriate.
External Reporting Requirements
Bleep will disclose financial conflicts of interest as required by applicable laws or regulations. Before expending any funds under a PHS award, Bleep will ensure public accessibility by posting financial conflicts of interest information on a publicly available web site or by responding in a timely manner to written requests as required under the regulations. The Designated Official will also report to the PHS Awarding Component, as detailed in the regulations, the existence of any financial conflict of interest that has not been eliminated and will ensure that Bleep has implemented a plan to manage the conflict.
If a financial conflict of interest is identified after its initial reporting and during ongoing research (e.g., through participation of a new Investigator) and has not been eliminated, Bleep will provide the PHS Awarding Component with an update within 60 days and ensure that it has implemented a plan to manage the conflict. If the financial conflicts of interest report involves a significant financial interest that was not disclosed by an Investigator or not previously reviewed or managed by Bleep (e.g., not reviewed or reported by a subrecipient in a timely manner), Bleep will undertake a retrospective review. Such retrospective review will determine whether there was bias in the design, conduct, or reporting of the PHS-funded research, or portion thereof, conducted prior to the identification and management of the conflict. If bias is found, Bleep will promptly notify the PHS Awarding Component and submit a mitigation report. Upon request, Bleep will provide HHS with information relating to any Investigator disclosure of significant financial interests; Bleep’s review of, and response to, such disclosure; and whether the disclosure resulted in Bleep’s determination of a financial conflict of interest.
Confidentiality
Bleep will, to the extent possible, protect the confidentiality of disclosures. In every instance, Bleep will endeavor to balance the privacy interests of individuals with its responsibility and obligation to identify and manage conflicts of interest. Disclosures will be available to Bleep staff only on a need-to-know basis and will not be disclosed outside of Bleep unless necessary to comply with contractual, legal, or regulatory requirements.
Investigator Noncompliance
If an Investigator knowingly fails to comply with this policy (e.g., fails to identify an actual or potential financial conflict of interest), Bleep may take appropriate disciplinary action, which may include, without limitation, termination of the Investigator’s participation in the research. In addition, for PHS-funded research, failure to comply with this policy or the applicable regulations shall result in the following:
- If the Investigator’s failure to comply with this policy or a financial conflicts of interest management plan has biased the design, conduct, or reporting of the PHS-funded research, Bleep shall promptly notify the PHS Awarding Component of the corrective action taken or to be taken;
- Bleep will make available to HHS all records pertinent to financial conflicts of interest and the management of those conflicts; and
- If HHS determines that a clinical PHS-funded research project whose purpose is to evaluate the safety or effectiveness of a drug, medical device, or treatment has been designed, conducted, or reported by an Investigator with a financial conflict of interest that was neither disclosed nor managed, Bleep shall require disclosure of the conflicting interest in each public presentation of the results of the research and shall request an addendum to previously published presentations, if necessary.
Training and Education
Investigators receive training to promote objectivity in research and to ensure Investigator compliance with regard to the applicable regulations and significant financial interest disclosure obligations. The training module and other resources developed by NIH will be updated as appropriate and can be accessed through the NIH Web site.
Bleep requires Investigators to complete such training every 4 years, and when any of the following occurs:
- Prior to engaging in research related to any PHS-funded grant
- Bleep revises its financial conflicts of interest policy or procedures in any manner that affects the Investigator’s obligations;
- An Investigator is new to Bleep; or
- Bleep finds that an Investigator is not in compliance with this policy or a financial conflicts of interest management plan.
Retention of Records
The Designated Official will retain financial conflicts of interest disclosure forms and other supporting information consistent with Bleep’s Record Retention policy. For PHS-funded research, records of all financial disclosures, whether or not they result in a reporting obligation, and all actions taken by Bleep with respect to each financial conflict of interest will be retained for at least 3 years from the date of submission of the final expenditures report or final payment on the contract or, where applicable, from other dates specified in 45 C.F.R. 75.361.
Point of Contact
If you have a conflict of interest or if you have a question to discuss, contact the Bleep Administrator at FCOI at [email protected]
If you would like to request information about FCOIs related to this study, please send a written request to the Bleep Administrator at [email protected]. Requests will be responded to within 5 business days.
Get your DreamPortTM Sleep Solution
for around a $1 day.
See what a difference good sleep can make!